NiCo2S4 Bi-metal Sulfide Coating using RAM for Solid-State Li-ion Batteries
The future of lithium-ion batteries may be with all solid-state lithium batteries (ASSLB), which avoid the use of flammable organic liquid electrodes. Replacing those electrodes with solid materials is safer. How to make the best version of an ASSB was explored in a study published by a team of researchers from the University of Ulsan in South Korea.
Researchers started with the cathode NCM622 (LiNi0.6Co0.2Mn0.2O2), popular for its energy density and operating voltage. But this material can degrade rapidly, and a partner material was sought. The researchers chose to use NiCo2S4, a stable superconductor material that doesn’t consume lithium ions. Instead, it stores ions chemically, providing them for later use.
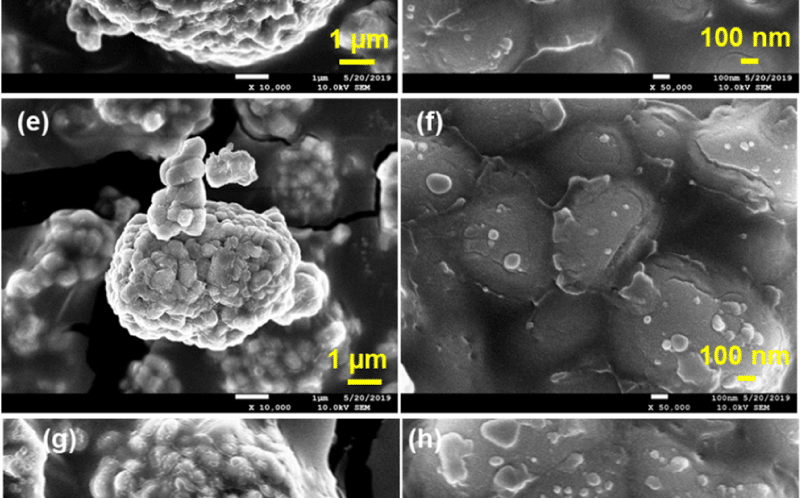
A key step in combining these materials was coating the NCM622 with NiCo2S4 using ResonantAcoustic Mixing©. The materials were loaded into a zirconia container and vibrated on a LabRAM II at 60 G for 20 minutes. Clusters of NiCo2S4 were continually broken up to disperse nanoparticles that would coat the surface of the NCM662.
This coated material was mixed with Li7P2S8I as a solid electrolyte and super-P carbon black. The solid electrode pellets were made by spreading cathode composite on one side of a piece of solid electrolyte. Then sheets of indium foil were placed, one to a side, where they were used as current collector and anode material. Indium was used because it is highly stable with sulfide-based solid electrodes, despite indium foil’s plateau of 0.62 V.
This coating would be key in the process, as bare NCM662 degrades rapidly in use. The coatings were tested with bare NCM622 and coatings of 0.1, 0.3 and 0.5 wt% NiCo2S4. All the coated materials showed improved specific capacity and reduced interfacial resistance over the bare NCM622.
“Especially, 0.3 wt% NiCo2S4-coated NCM622 exhibited a capacity retention of 60.6% at a current density of 15 mA/g for 20 cycles, compared to only 37.3% for bare NCM622. Finally, interfacial XPS and transmission electron microscopy-electron energy loss spectroscopy analyses confirmed the stable state of 0.3 wt% NiCo2S4-coated NCM622 with minimal side reactions,” the researchers wrote.
The bare NCM622 area had almost no lithium and very little phosphorous and sulfur. Researchers considered that this was because there were significant side reactions and that having no coating damaged the cathode interface.
“NCM622 coated with 0.3 wt% NiCo2S4 showed strong S 2p (162.1, 163.0 eV, respectively) and −O–S– S 2p (163.0, 164.1 eV, respectively) peaks, possibly because of the minimal side reactions induced by the bimetal sulfide coating to satisfy the maximum compatibility of the sulfide-based solid electrolyte,” researchers wrote. The researchers focused on the 0.3 wt% material for this study, but they looked at other concentrations.
“Further increasing the NP concentration to 0.5 wt% produced a thicker and denser coating. This nickel cobalt sulfide NP modification may have decreased the interfacial resistance, although it may also have obstructed the lithium-ion transfer pathway. Therefore, reducing the nanomaterial size to approximately 10 nm may further improve the surface-modified NPs and their physical or electrochemical qualitie the researchers wrote.
The researchers considered the project a success.
“These results indicate that NiCo2S4 coating using the thermal decomposition with the resonant acoustic coating technique can minimize the side reaction between the cathode and sulfide-based solid electrolyte, thus improving the capacity of ASSLBs. Thus, we concluded that the NiCo2S4 NPs coating provided improved specific capacity and reduced interfacial resistance than bare NCM622,” they wrote.
Sources:




